getDesignGroupSequential()
getDesignInverseNormal()
getDesignFisher()
getDesignConditionalDunnett()Introduction to the rpact Package
January 14, 2026
The rpact Package 📦
- Comprehensive validated R package implementing methodology described in Wassmer and Brannath (2016)
- Enables the design of traditional and confirmatory adaptive group sequential designs
- Provides interim data analysis and simulation including early efficacy stopping and futility analyses
- Enables sample-size reassessment with different strategies
- Enables treatment arm selection in multi-stage multi-arm (MAMS) designs
- Enables subset selection in population enrichment designs
- Provides a comprehensive and reliable sample size calculator

Developed by RPACT 🏢
- RPACT GbR company founded in 2017 by Gernot Wassmer and Friedrich Pahlke
- Idea: open source development with help of “crowd funding”
- Currently supported by 21 companies
- \(>\) 80 presentations and training courses since 2018, e.g., FDA in March 2022
- 30 vignettes based on Quarto and published on rpact.org/vignettes
- 28 releases on CRAN since 2018



RCONIS 🚀
- Grow RPACT company to offer a wider range of services
- Statistical consulting and engineering services: Research Consulting and Innovative Solutions
- Joint venture between RPACT GmbH (rpact.com) and inferential.biostatistics GmbH (inferential.bio) founded by Daniel Sabanés Bové and Carrie Li
- Website: rconis.com

Second Edition of the Book

- Provides up-to-date overview of group sequential and confirmatory adaptive designs in clinical trials
- Describes available software including R packages and has
rpactcode examples - Supplemented with a discussion of practical applications
- Published Oct 2025
Second Edition of Jennison and Turnbull


The R Package rpact – Functional Range

Trial Designs 🔬
- Fixed sample designs:
- continuous, binary, count, survival outcomes
- Group sequential designs:
- efficacy interim analyses, futility stopping, alpha-spending functions
- Adaptive designs:
- Inverse normal and Fisher’s combination test, conditional error rate principle
- Provides adjusted confidence intervals and bias corrected estimates
- Multi-arm multi-stage (MAMS) and enrichment designs, sample size reassessment
Easy to understand R commands:
Sample Size and Power Calculation 💻
Sample size and power can be calulcated for testing:
- means (continuous endpoint)
- proportions (binary endpoint)
- hazards (survival endpoint)
- Note: flexible recruitment and survival time options
- rates (count endpoint)
Example: Sample Size Calculation 🧮
Sample size calculation for a continuous endpoint
Sequential analysis with a maximum of 3 looks (group sequential design), one-sided overall significance level 2.5%, power 80%. The results were calculated for a two-sample t-test, H0: mu(1) - mu(2) = 0, H1: effect = 2, standard deviation = 5.
| Stage | 1 | 2 | 3 |
|---|---|---|---|
| Planned information rate | 33.3% | 66.7% | 100% |
| Cumulative alpha spent | 0.0001 | 0.0060 | 0.0250 |
| Stage levels (one-sided) | 0.0001 | 0.0060 | 0.0231 |
| Efficacy boundary (z-value scale) | 3.710 | 2.511 | 1.993 |
| Futility boundary (z-value scale) | 0 | 0 | |
| Efficacy boundary (t) | 4.690 | 2.152 | 1.384 |
| Futility boundary (t) | 0 | 0 | |
| Cumulative power | 0.0204 | 0.4371 | 0.8000 |
| Number of subjects | 69.9 | 139.9 | 209.8 |
| Expected number of subjects under H1 | 170.9 | ||
| Overall exit probability (under H0) | 0.5001 | 0.1309 | |
| Overall exit probability (under H1) | 0.0684 | 0.4202 | |
| Exit probability for efficacy (under H0) | 0.0001 | 0.0059 | |
| Exit probability for efficacy (under H1) | 0.0204 | 0.4167 | |
| Exit probability for futility (under H0) | 0.5000 | 0.1250 | |
| Exit probability for futility (under H1) | 0.0480 | 0.0035 |
Legend:
- (t): treatment effect scale
Adaptive Analysis 📈
Perform interim and final analyses during the trial using group sequential method or p-value combination test (inverse normal or Fisher)
Calculate adjusted point estimates and confidence intervals (cf., Robertson et al. (2023), Robertson et al. (2025))
Perform sample size reassessment using the observed data, e.g., based on calculation of conditional power
Easy to understand R commands:
Some highlights:
- Automatic boundary recalculations during the trial for analysis with alpha spending approach, including under- and over-running
- Adaptive analysis tools for multi-arm trials and enrichment designs
Simulation Tool 🧪
Obtain operating characteristics of different designs:
- Assessment of adaptive sample size recalculation strategies
- Assessment of treatment selection strategies in multi-arm trials
- Assessment of population selection strategies in enrichment designs
Easy to understand R commands:
Example:
\(\rightarrow\) rpact useful for conducting flexible simulations in clinical trial planning
RPACT Cloud
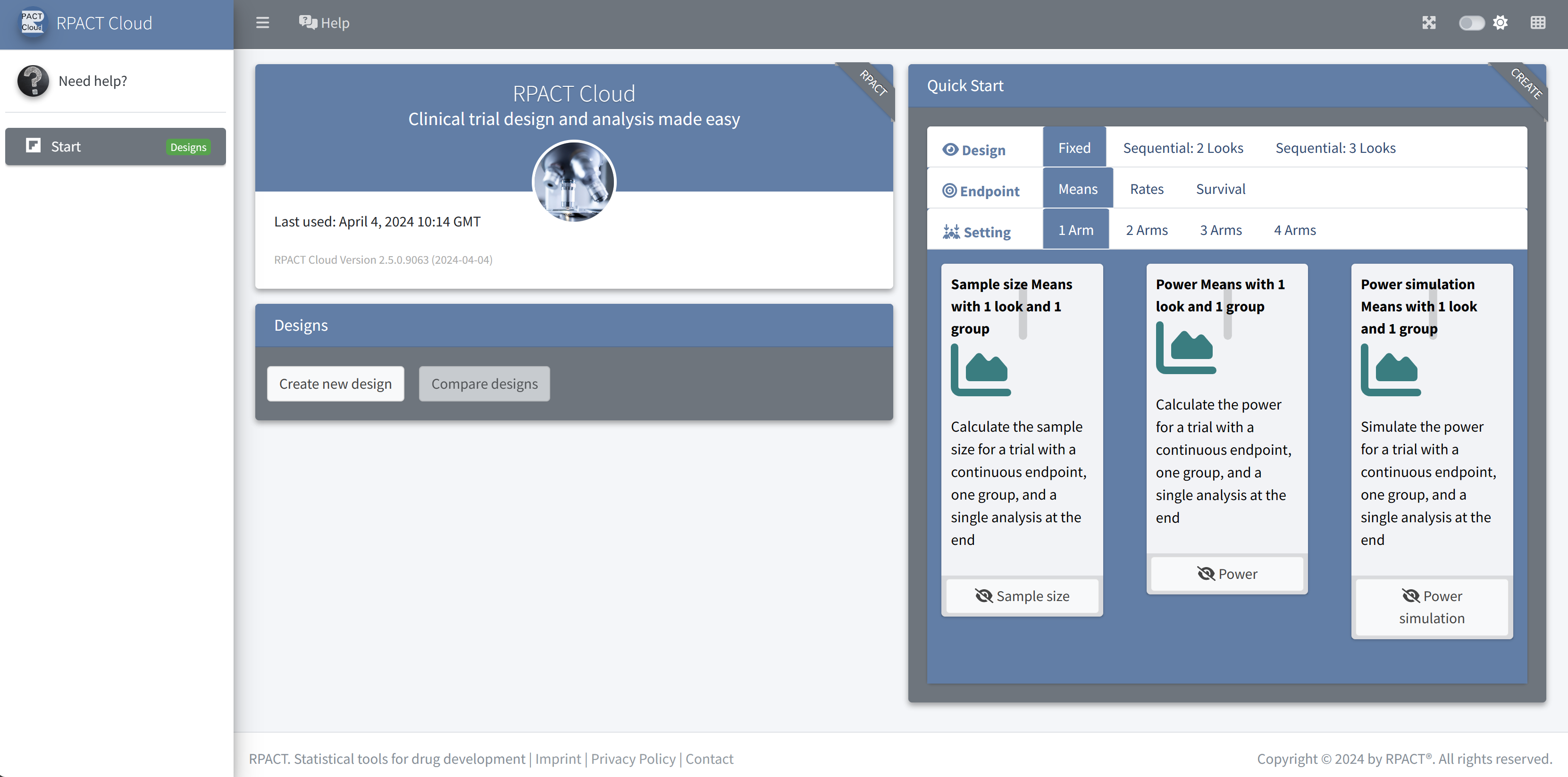
RPACT Cloud ☁️
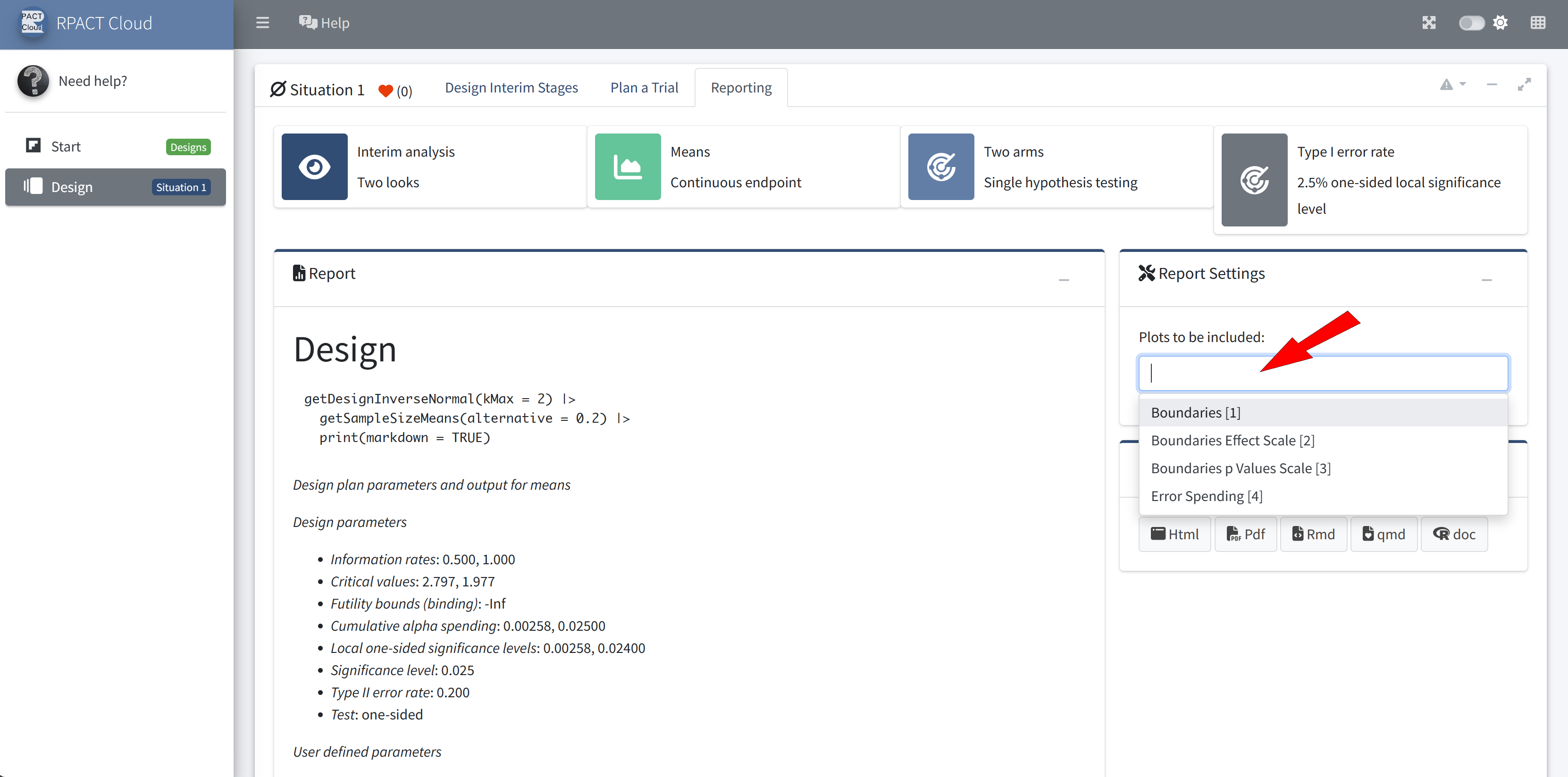
Graphical user interface
Web based usage without local installation on nearly any device
Provides an easy entry to to learn and demonstrate the usage of
rpactStarting point for your R Markdown or Quarto reports
Online available at rpact.cloud
Start Page
Design
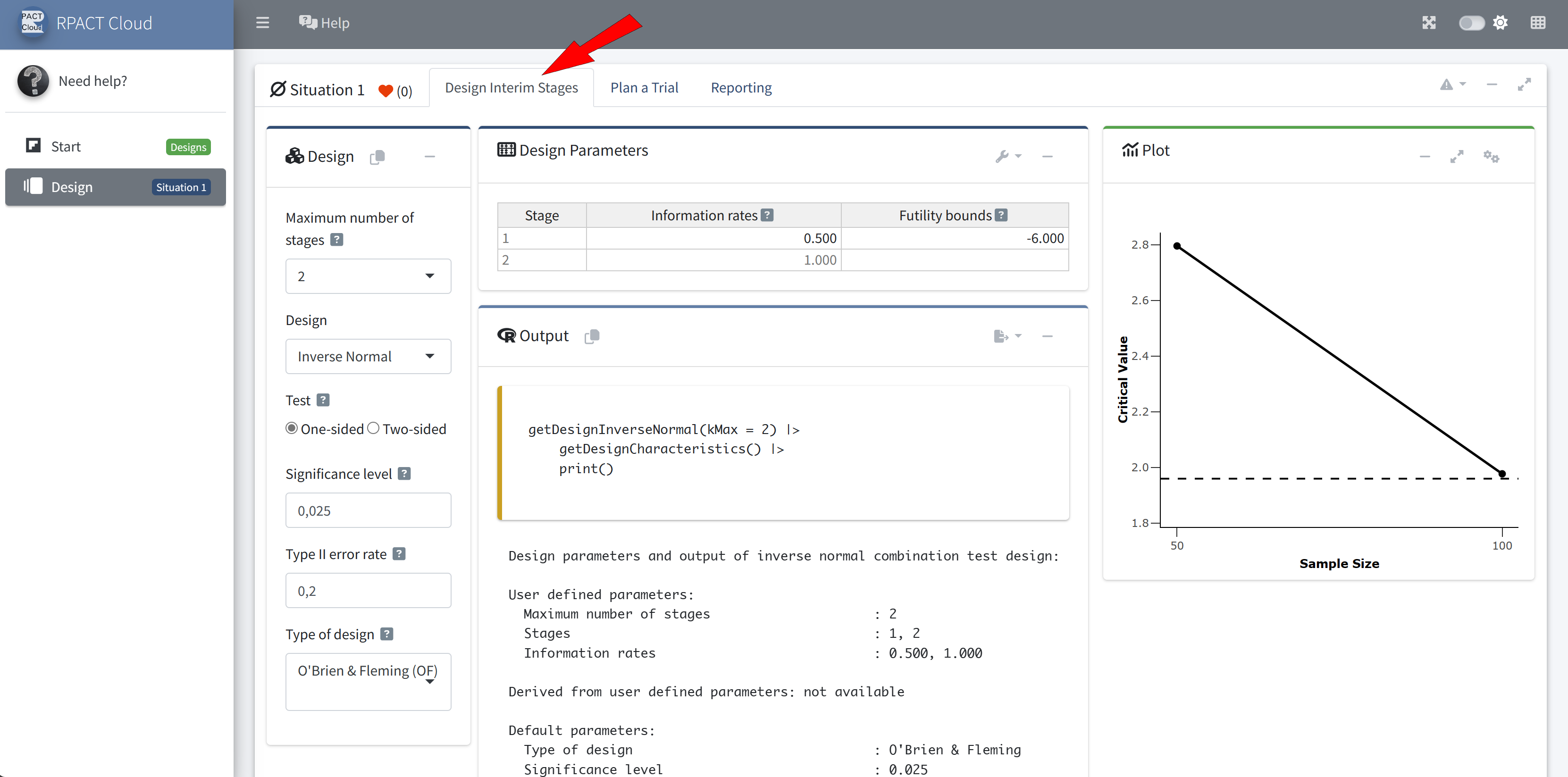
Reporting